Metallic bonds are formed between like metal ions. Ionic bonds are formed between a cation (usually a metal) and an anion (usually a nonmetal).
Both metals and salts are arranged in crystals. As a result, both metals and salts have high melting and boiling points.
Metals conduct electricity even as solids. Ionic compounds only conduct electricity when they are molten or dissolved in water.
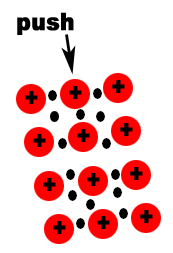
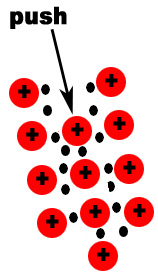
Metals and salts have different properties because in a metal valence electrons are not attached to any one atom; in a salt they are. So, in metals, valence electrons are free to move around freely throughout the crystal. These free moving electrons are why solid metals can conduct electricity and ionic compounds cannot.
Metals are malleable and ductile. Ionic compounds are brittle. Although atoms in a metal are in a somewhat fixed position, they can move without breaking the attraction between atoms. This allows them to be malleable and ductile. If the atoms in an ionic compounds move, like charges line up and the repulsion between the atoms cause the ionic compound to break apart. The diagram below shows how metals respond to a force.
|
|